Abstract
Background: Despite major advances in the treatment of multiple myeloma (MM) only a minority of patients achieve long-term disease control. Immunotherapy combined with autologous hematopoietic stem cell transplantation (auto-HCT) may reduce relapse rates. Vaccination with Ig idiotype (Id) conjugated with a carrier protein, keyhole limpet hemocyanin (KLH), was associated with superior disease-free survival in a randomized trial in follicular lymphoma (Schuster S. et al. JCO 2011), and Id is also a tumor-specific antigen in MM. In addition, anti-CD3/-CD28 costimulated adoptive T-cell transfer augmented immune responses to pneumococcal vaccination despite cytotoxic therapy (Rapoport A et al. Nat Med 2005). We therefore hypothesized that Id-KLH vaccine + the vaccine-primed costimulated T cells will result in a robust Id-specific humoral and cellular response, compared with control vaccine (KLH only).
Methods: In this randomized, phase II trial, eligible patients were randomized 1:1 to receive either KLH-only or Id-KLH vaccine, followed by auto-HCT, and then vaccine- primed costimulated T cells followed by two booster doses of the vaccine to which they were randomized. Immune response (IR) was evaluated by gene expression profiling (GEP) using NanoString nCounter.
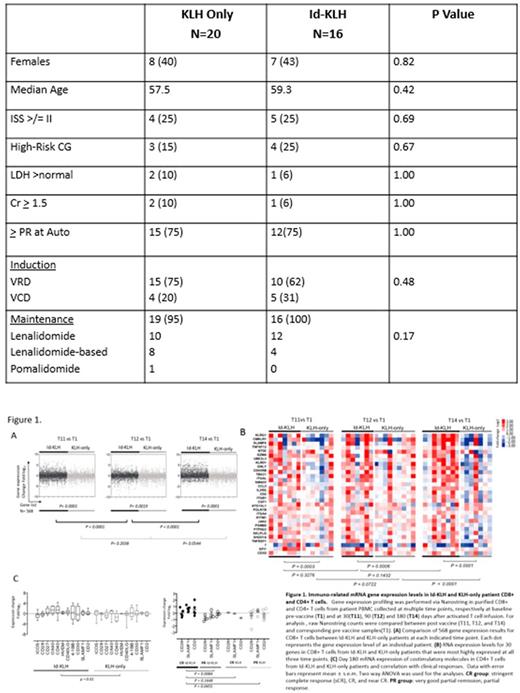
Results: A total of 36 patients were enrolled between 1/2013 and 5/2015, 20 to KLH-only and 16 to Id-KLH arm, and completed their assigned treatments. There were no significant differences between the groups in age, gender, stage, cytogenetic risk, induction regimen or response to induction (Table). Nineteen (95%) and 16 (100%) patients received maintenance therapy after protocol treatment in KLH-only and Id-KLH, respectively (p=0.42). No infusion reactions or dose-limiting toxicity was seen in either arm. There was no non-relapse mortality at 100-day and 1-year in either arm. At last evaluation, 5 (25%) and 7 (44%) had achieved CR in KLH-only and Id-KLH, respectively (p=0.29). Six (30%) and 9 (56%) achieved at least nCR in KLH-only and Id-KLH, respectively (p=0.17), and 12 (60%) and 12 (75%) achieved at least a VGPR in KLH-only and Id-KLH, respectively (0.48). Ten (50%) and 8 (50%) patients, for whom there were sufficient samples available underwent IR analysis in KLH-only and Id-KLH, respectively at baseline and at 30, 90 and 180 days after activated T cell infusion. In an initial 568 gene panel analyzed for IR, compared with KLH-only, there was a greater change in IR genes in CD8+ T cells in Id-KLH at days 30, 90 and 180 (Figure 1A). In a 30-gene panel of the most upregulated IR genes in CD8+ cells, there was a significantly higher expression of these genes in Id-KLH at all post-treatment time points (Figure 1B). Specifically, at day 180 post-treatment, there was higher expression of immune co-stimulatory genes in Id-KLH in both CD8+ and CD4+ T cell subsets. This increased co-stimulatory gene expression in Id-KLH vaccinated CD4+ (Figure 1C) and CD8+ T cells (not shown) was most marked in patients who had achieved a CR. After a median follow up of 38.3 months, 3-year PFS in KLH-only and Id-KLH was 64% and 53%, respectively (p=0.25).
Conclusion: Id-KLH vaccine and vaccine-primed costimulated T cells can be safely administered in the setting of auto-HCT. In this randomized trial we detected a trend towards a deeper clinical response in the Id-KLH arm. There were significantly more robust IR in CD4+ and CD8+ T cells in the Id-KLH arm,which also correlated withdepth of clinical response. Future trials should focus on selection of patients most likely to mount a robust IR to Id vaccines.
Shah: TeneoBio, Inc.: Honoraria; Indapta Therapeutics: Other: Stock Ownership; Celgene: Research Funding; Celgene, Indapta Therapeutics, Takeda: Consultancy. Patel: Juno: Consultancy; Celgene: Consultancy; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding. Thomas: Bristol Myers Squibb: Research Funding; Celgene: Research Funding. Orlowski: BioTheryX: Consultancy, Membership on an entity's Board of Directors or advisory committees. Cohen: Celgene: Consultancy; Bristol Meyers Squibb: Consultancy, Research Funding; GlaxoSmithKline: Consultancy; Janssen: Consultancy. Vogl: Constellation: Research Funding; Karyopharm: Consultancy; GSK: Research Funding; Calithera: Research Funding; Takeda: Consultancy, Research Funding; Teva: Consultancy; Amgen: Consultancy; Celgene: Consultancy. June: Immune Design: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Novartis: Patents & Royalties, Research Funding; Tmunity Therapeutics: Equity Ownership, Research Funding; WIRB/Copernicus Group: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celldex: Honoraria, Membership on an entity's Board of Directors or advisory committees. Kwak: Celltrion, Inc: Consultancy; InnoLifes: Consultancy, Equity Ownership; Pepromene Bio: Consultancy, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal